


Sharpsafe - simplifying clinical waste through easily identifiable sharps containers.
Worldwide needlestick injuries are among the most common occupational hazards for healthcare workers.
The WHO states that 2 million occupational exposures to shares injuries annually1, and at least 20 different pathogens are transmitted by a needlestick injury.2
Our Sharpsafe brand is here to help protect you.

We have five different ranges to suit your needs:

Pocket Range - 0.2L/0.45L/0.6L
Designed to fit perfectly in a pocket or bag. It can be used on persons such as ambulance service, community nurses, personal travel, and police.

Small - 0.8L/1L/1.8L/2L/3L
Designed for medium usage and community setting, perfect for home use, community nurses, dentists, and ambulance service.

Medium - 4L/7L
Designed for accident and emergency wards, GP surgeries, general medicine wards, dental practices, and care home settings.

Large - 9L/13L/24L/30L
Designed for accident and emergency wards, phlebotomy wards, blood banks, care home settings, and oncology centres.
Sharpsafe Features and Benefits
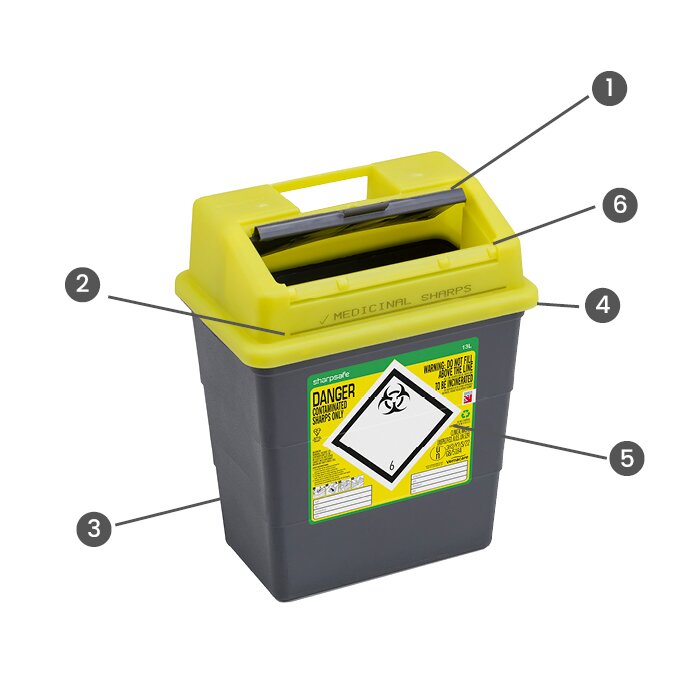
(1) One Click Temporary Closure
Provides added reassurance when the container is in use and helps to prevent sharps injuries by restricting access.
(2) Clearly Visible Fill Line & Semi Translucent Lid
Easy to see and helps prevent overfilling, protecting healthcare workers and patients from potential needlestick injury.
(3) Low Carbon Polypropylene
Prevents sharps penetration through the wall of the container protecting healthcare workers and patients from potential needlestick injury.
(4) Four Click Assembly
An audible click confirms the lid and base are assembled correctly, indicating it is safe to use.
(5) In-mould label
In-mould label is moulded to the wall of the container, they cannot be removed and offer a permanent audit trail.
(6) Final closure
Permanently closes the container so no further waste can be added, preventing overfilling and potential spillages.
Plus, Safety Shield
A non-return flap built into the lid of every mid size container to reduce risk of overfilling.
And Fully Compliant with ISO23907-1:2019 and UN3291

Committed to Sustainability
We are committed to helping you reduce your carbon footprint. Our Sharpsafe containers have a 94% reduction in their carbon footprint when compared to containers using virgin plastic.
Our range combines high-performance sharps waste management with our eco-conscious manufacturing. By switching our manufacturing over to our low carbon recycled materials, we are helping to support healthcare facilities to achieve their Net Zero targets.
Get in touch with our expert team
We can offer you the optimal solution for clinical waste management in all healthcare settings. Speak to our team today to start the journey.
Contact us
